Company Expects New Personal Medical Device Kit to be available to customer by early 2022
REDWOOD CITY, CA / ACCESSWIRE / September 14, 2021 / Biotricity Inc. (NASDAQ:BTCY), a medical diagnostic and consumer healthcare technology company, today announced that it has developed a personal medical device kit, Biokit, a bundled home-use set comprised of a digital thermometer, a pulse oximeter, and a blood pressure cuff. All three devices are FDA cleared, wireless capable and integrate into the Biotricity ecosystem, a platform for interconnectivity. This is the next step in the evolution of the Bioflux solution, where other available tools are fully integrated to create a complete solution for remote cardiac monitoring.
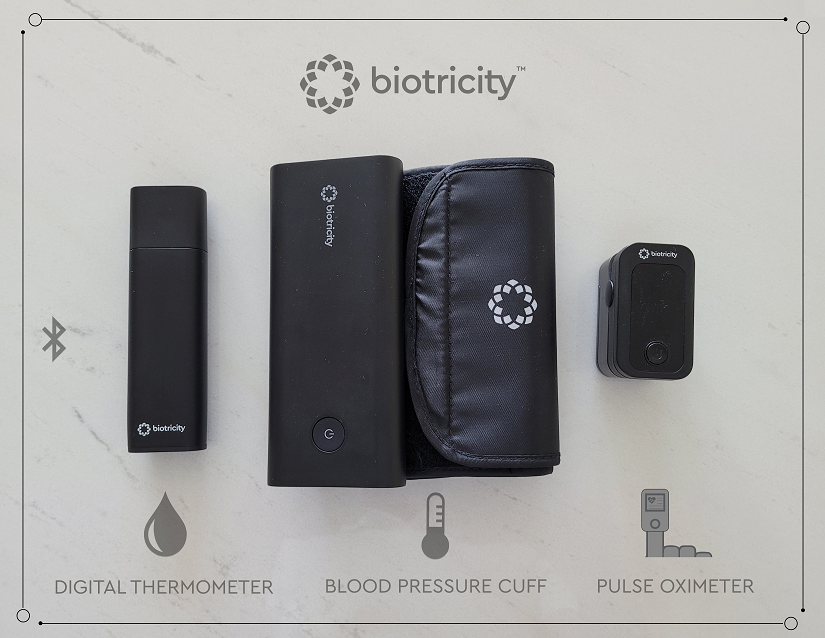
The kit is in the final production stage before commercial launch, and the Company expects each device within the kit to be available to customers for the first quarter of 2022. With all devices being FDA cleared, the company does not anticipate any additional submissions to the FDA prior to commercial launch.
"We created the Biokit to strengthen our long-term vision of supporting diagnosed cardiac patients throughout their cardiac health journey," Waqaas Al Siddiq, Biotricity CEO explained. "Biokit is a natural extension of our Bioflux cardiac-diagnostic offering. For patients with cardiac disease, a personal medical device kit is ideal for long term monitoring. After speaking with both patients and physicians, it was clear that a product like this was an immediate need. I am pleased with how quickly we have been able to develop a solution to fulfill this customer feedback, which is a testament to the team here. The Biokit will expand our total addressable market with a tailored offering for the $18.4 billion-dollar North American portable medical device market1. With Bioflux and Biokit combined, Biotricity will soon offer a comprehensive cardiac diagnostics and management product line for effective, accurate and economic long-term disease management."
The Biokit is a wirelessly connected personal medical device kit that was developed to address the challenges with existing home-based medical devices. It includes the following devices, all of which integrate with the Biotricity ecosystem and provide near real-time data to both the physician and the patient through the web and an App:
- Biokit BP - A wireless digital blood pressure cuff enabling patients to collect BP data.
- Biokit TP - A wireless digital thermometer enabling patients to collect temperature data.
- Biokit 02 - A wireless digital pulse oximeter enabling patients to collect blood-oxygen data.
- Biotricity Ecosystem Integration - All devices within the Biokit are seamlessly integrated into the Biotricity ecosystem, enabling patients to track and store their data. Most importantly, this data is also stored and available for physician interim review and for follow up visits.
About Biotricity Inc.
Biotricity is reforming the healthcare market by bridging the gap in remote monitoring and chronic care management. Doctors and patients trust Biotricity's unparalleled standard for preventive & personal care, including diagnostic and post-diagnostic solutions for chronic conditions. The Company develops comprehensive remote health monitoring solutions for the medical and consumer markets. To learn more, visit www.biotricity.com.
Important Cautions Regarding Forward-Looking Statements
Any statements contained in this press release that do not describe historical facts may constitute forward-looking statements. Forward-looking statements, which involve assumptions and describe our future plans, strategies, and expectations, are generally identifiable by use of the words "may," "should," "would," "will," "could," "scheduled," "expect," "anticipate," "estimate," "believe," "intend," "seek," "project," or "goal" or the negative of these words or other variations on these words or comparable terminology. Forward-looking statements may include, without limitation, statements regarding (i) the plans, objectives and goals of management for future operations, including plans, objectives or goals relating to the design, development and commercialization of Bioflux or any of the Company's other proposed products or services, (ii) a projection of income (including income/loss), earnings (including earnings/loss) per share, capital expenditures, dividends, capital structure or other financial items, (iii) the Company's future financial performance, (iv) the regulatory regime in which the Company operates or intends to operate and (v) the assumptions underlying or relating to any statement described in points (i), (ii), (iii) or (iv) above. Such forward-looking statements are not meant to predict or guarantee actual results, performance, events or circumstances and may not be realized because they are based upon the Company's current projections, plans, objectives, beliefs, expectations, estimates and assumptions and are subject to a number of risks and uncertainties and other influences, many of which the Company has no control over. Actual results and the timing of certain events and circumstances may differ materially from those described by the forward-looking statements as a result of these risks and uncertainties. Factors that may influence or contribute to the inaccuracy of the forward-looking statements or cause actual results to differ materially from expected or desired results may include, without limitation, the Company's inability to obtain additional financing, the significant length of time and resources associated with the development of its products and related insufficient cash flows and resulting illiquidity, the Company's inability to expand the Company's business, significant government regulation of medical devices and the healthcare industry, lack of product diversification, existing or increased competition, results of arbitration and litigation, stock volatility and illiquidity, and the Company's failure to implement the Company's business plans or strategies. These and other factors are identified and described in more detail in the Company's filings with the SEC. The Company assumes no obligation to update any forward-looking statements in order to reflect any event or circumstance that may arise after the date of this release.
Contacts:
Investor Relations:
Biotricity Inc.
1-800-590-4155
investors@biotricity.com
MKR Investor Relations, Inc.
Todd Kehrli or Mark Forney
btcy@mkr-group.com
Media Contact:
Bospar
prforbiotricity@bospar.com
SOURCE: Biotricity, Inc.
View source version on accesswire.com:
https://www.accesswire.com/663949/Biotricity-Unveils-Biokit-with-3-Personal-Medical-Devices-to-Expand-into-Cardiac-Disease-Management













