The Phase 3 ALPINE trial met the primary endpoint at interim analysis, with BRUKINSA showing superiority in investigator-assessed overall response rate versus ibrutinib
Early progression-free survival and overall survival data are supportive of findings in response rate
BRUKINSA demonstrated superiority in key secondary endpoint of atrial fibrillation or flutter and advantages in overall cardiac safety
Company to host investor call and webcast today at noon ET (18:00 CEST)
BeiGene, Ltd. (NASDAQ: BGNE; HKEX: 06160), a global biotechnology company focused on developing and commercializing innovative medicines worldwide, today presented results from the interim analysis of the Phase 3 ALPINE trial comparing its BTK inhibitor BRUKINSA® (zanubrutinib) to ibrutinib in adult patients with relapsed or refractory (R/R) chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), including superiority in the primary endpoint of investigator-assessed overall response rate (ORR) and superiority in a key secondary endpoint of atrial fibrillation or flutter.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210611005105/en/
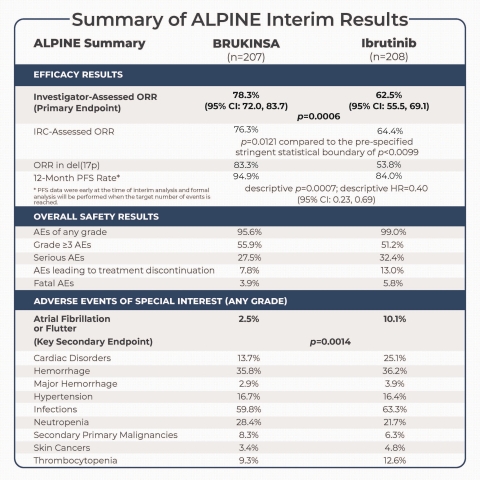
Summary of ALPINE Interim Analysis (Graphic: Business Wire)
These results were reported in an oral presentation as part of the Presidential Symposium and featured during the congress press briefing at the 26th European Hematology Association 2021 (EHA2021) Virtual Congress, taking place virtually on June 9-17, 2021.
“While ibrutinib has transformed the outlook for patients with chronic lymphocytic leukemia, or CLL, not all patients respond to treatment and toxicities associated with prolonged exposure remain an issue,” commented Peter Hillmen, MBChB, Ph.D., Professor of Experimental Haematology at University of Leeds and principal investigator of the ALPINE trial. “The ALPINE trial is the only head-to-head study in CLL to demonstrate an efficacy advantage for a more selective BTK inhibitor over ibrutinib. Compared to ibrutinib, BRUKINSA demonstrated superiority in investigator-assessed overall response rate in patients with relapsed or refractory CLL. Additionally, the event rate of atrial fibrillation or flutter, an important indicator of cardiac toxicity, was also significantly lower with BRUKINSA.”
“The positive data from the interim analysis of the ALPINE trial, including a superior overall response rate by investigator assessment, supportive initial data in progression-free survival and overall survival, and a significantly lower rate of atrial fibrillation of any grade, reinforce our belief that the differentiated profile of BRUKINSA can provide clinical benefits for patients with CLL,” said Jane Huang, M.D., Chief Medical Officer, Hematology at BeiGene. “As evidenced in ALPINE and ASPEN, our head-to-head trials of BRUKINSA against the first-generation BTK inhibitor ibrutinib, this potentially best-in-class molecule can provide meaningful responses and consistent safety advantages for these patients. In addition to ALPINE, we are evaluating BRUKINSA in the Phase 3 SEQUOIA trial in treatment-naïve CLL, and expect to share topline results as early as later this year.”
Interim Analysis of the ALPINE Trial Comparing BRUKINSA to Ibrutinib in R/R CLL
Oral presentation; Abstract code: LB1900
Results from the planned interim analysis presented at EHA were based on the first 415 patients enrolled in the ALPINE trial, including 207 on BRUKINSA treatment and 208 on ibrutinib treatment.
In the interim analysis, with a median follow-up time of 15.3 months, the trial met the primary endpoint with BRUKINSA demonstrating superiority in ORR, defined as the combined rate of complete responses (CRs) and partial responses (PRs), per investigator assessment. In the ORR analysis conducted by independent review committee (IRC), BRUKINSA demonstrated non-inferiority in the interim analysis. Efficacy results included:
- As assessed by investigator, BRUKINSA achieved an ORR of 78.3% (95% CI: 72.0, 83.7), a statistically significant improvement compared to 62.5% (95% CI: 55.5, 69.1) with ibrutinib (p=0.0006);
- As assessed by IRC, BRUKINSA achieved an ORR of 76.3%, numerically higher but not statistically significant compared to 64.4% with ibrutinib (p=0.0121 compared to the pre-specified stringent statistical boundary of p<0.0099 set for the interim analysis);
- In patients whose tumor exhibited chromosome 17p deletion (del[17p]), the ORR was 83.3% in the BRUKINSA arm, compared to 53.8% in the ibrutinib arm, as assessed by investigator;
- PFS data were early at the time of interim analysis and formal analysis will be performed when the target number of events is reached. The PFS rate at 12 months was 94.9% in the BRUKINSA arm, compared to 84.0% in the ibrutinib arm (descriptive p=0.0007; descriptive hazard ratio [HR]=0.40 [95% CI: 0.23, 0.69]), as assessed by investigator; and
- OS data were early at the time of interim analysis. The OS rate at 12 months was 97.0% in the BRUKINSA arm, compared to 92.7% in the ibrutinib arm (descriptive p=0.1081; descriptive HR=0.54 [95% CI: 0.25, 1.16]).
In the interim analysis, the ALPINE trial also met a pre-specified key secondary endpoint related to safety, with BRUKINSA demonstrating a statistically significant lower risk of atrial fibrillation or flutter and advantages in the overall cardiac safety profile, compared to ibrutinib. Treatment discontinuation was more common in the ibrutinib arm. Safety results included:
- 195 patients (95.6%) in the BRUKINSA arm experienced at least one adverse event (AE) of any grade, compared to 205 patients (99.0%) in the ibrutinib arm, and the most common (≥10%) AEs included anemia (BRUKINSA vs. ibrutinib: 13.2% vs. 15.0%), arthralgia (9.3% vs. 14.0%), contusion (10.3% vs. 8.7%), cough (12.7% vs. 6.3%), diarrhea (16.7% vs. 19.3%), hypertension (15.7% vs. 13.0%), muscle spasm (2.9% vs. 11.1%), neutropenia (19.6% vs. 15.5%), upper respiratory tract infection (21.6% vs. 14.0%), and urinary tract infection (10.8% vs. 8.2%);
- 114 patients (55.9%) in the BRUKINSA arm experienced Grade ≥3 AEs, compared to 106 patients (51.2%) in the ibrutinib arm;
- 56 patients (27.5%) in the BRUKINSA arm experienced serious AEs, compared to 67 patients (32.4%) in the ibrutinib arm;
- Dose reduction and interruption due to AEs occurred in 23 patients (11.3%) and 81 patients (39.7%) in the BRUKINSA arm, respectively, compared to 25 patients (12.1%) and 84 patients (40.6%) in the ibrutinib arm;
- 16 patients (7.8%) discontinued BRUKINSA treatment due to AEs, with none caused by cardiac disorders; in comparison, 27 patients (13.0%) discontinued ibrutinib treatment due to AEs, with seven caused by cardiac disorders, including two of atrial fibrillation, and one each of cardiac arrest, cardiac failure, myocardial infarction, palpitations, and ventricular fibrillation;
- Fatal AEs were reported in eight patients (3.9%) in the BRUKINSA arm, compared to 12 patients (5.8%) in the ibrutinib arm;
- A key secondary endpoint of atrial fibrillation or flutter of any grade occurred in five patients (2.5%) in the BRUKINSA arm, significantly lower than the 21 patients (10.1%) in the ibrutinib arm (p=0.0014);
- Grade ≥3 atrial fibrillation or flutter occurred in two patients (1.0%) in the BRUKINSA arm, compared to four patients (1.9%) in the ibrutinib arm;
- Additional AEs of special interest of any grade included cardiac disorders (BRUKINSA vs. ibrutinib: 13.7% vs. 25.1%), hemorrhage (35.8% vs. 36.2%), major hemorrhage (2.9% vs. 3.9%), hypertension (16.7% vs. 16.4%), infections (59.8% vs. 63.3%), neutropenia (28.4% vs. 21.7%), secondary primary malignancies (8.3% vs. 6.3%), skin cancers (3.4% vs. 4.8%), and thrombocytopenia (9.3% vs. 12.6%); and
- Grade ≥3 AEs of special interest included cardiac disorders (BRUKINSA vs. ibrutinib: 2.5% vs. 6.8%), hemorrhage (2.9% vs. 2.9%), major hemorrhage (2.9% vs. 2.9%), hypertension (10.8% vs. 10.6%), infections (12.7% vs. 17.9%), neutropenia (18.6% vs. 15.0%), secondary primary malignancies (4.9% vs. 1.9%), skin cancers (1.5% vs. 1.0%), and thrombocytopenia (3.4% vs. 3.4%).
Summary of ALPINE Interim Results |
||||
ALPINE
|
|
BRUKINSA
|
|
Ibrutinib
|
Efficacy Results |
||||
Investigator-
|
|
78.3%
|
|
62.5%
|
|
p=0.0006 |
|||
IRC-Assessed ORR |
|
76.3% |
|
64.4% |
p=0.0121 compared to the pre-specified stringent
|
||||
ORR in del(17p) |
|
83.3% |
|
53.8% |
12-Month PFS Rate* |
94.9% |
|
84.0% |
|
descriptive p=0.0007;
|
||||
* PFS data were early at the time of interim analysis and formal analysis will be performed when the target number of events is reached. |
||||
Overall Safety Results |
||||
AEs of any grade |
|
95.6% |
|
99.0% |
Grade ≥3 AEs |
|
55.9% |
|
51.2% |
Serious AEs |
|
27.5% |
|
32.4% |
AEs leading to treatment discontinuation |
|
7.8% |
|
13.0% |
Fatal AEs |
|
3.9% |
|
5.8% |
Adverse Events of Special Interest (Any Grade) |
||||
Atrial Fibrillation
|
|
2.5% |
|
10.1% |
p=0.0014 |
||||
Cardiac Disorders |
|
13.7% |
|
25.1% |
Hemorrhage |
|
35.8% |
|
36.2% |
Major Hemorrhage |
|
2.9% |
|
3.9% |
Hypertension |
|
16.7% |
|
16.4% |
Infections |
|
59.8% |
|
63.3% |
Neutropenia |
|
28.4% |
|
21.7% |
Secondary Primary Malignancies |
|
8.3% |
|
6.3% |
Skin Cancers |
|
3.4% |
|
4.8% |
Thrombocytopenia |
|
9.3% |
|
12.6% |
To learn more about BeiGene’s research and development and activities around EHA2021, please visit https://beigenemedical.eu.
BeiGene EHA2021 Investor Conference Call and Webcast Information
BeiGene will host an investor and analyst conference call and webcast to discuss results from the interim analysis of the ALPINE trial, other data presented at EHA2021, and the BRUKINSA clinical program, today Friday, June 11, at 12:00 p.m. (noon) ET (18:00 CEST).
A live webcast of the conference call can be accessed from the investors section of BeiGene’s website at http://ir.beigene.com or http://hkexir.beigene.com. An archived replay will be available two hours after the event for 90 days.
About Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma
Chronic lymphocytic leukemia (CLL) is the most common form of leukemia in adults, with a global incidence of approximately 114,000 new cases in 2017.1,2 CLL affects white blood cells or lymphocytes in the bone marrow.1 Proliferation of cancer cells (leukemia) in the marrow result in reduced ability to fight infection and spread into the blood, which affects other parts of the body including the lymph nodes, liver and spleen.1,3 The BTK pathway is a known route that signals malignant B cells and contributes to the onset of CLL.4 Small lymphocytic lymphoma (SLL) is a non-Hodgkin’s lymphoma affecting the B-lymphocytes of the immune system, which shares many similarities to CLL but with cancer cells found mostly in lymph nodes.5
About ALPINE
ALPINE is a randomized, global Phase 3 trial (NCT03734016) comparing BRUKINSA against ibrutinib in previously treated patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
In the trial, a total of 652 patients were randomized into two arms, with the first receiving BRUKINSA (160 mg orally twice daily) and the second receiving ibrutinib (420 mg orally once daily) until disease progression or unacceptable toxicity. The primary analysis of overall response rate (ORR), defined by pre-specified non-inferiority of BRUKINSA versus ibrutinib, was assessed by investigator and independent review committee (IRC) using the modified 2008 iwCLL guidelines, with modification for treatment-related lymphocytosis for patients with CLL, and per Lugano Classification for non-Hodgkin’s lymphoma for patients with SLL. There was hierarchical testing of non-inferiority followed by superiority in ORR as assessed by investigator and IRC. Key secondary endpoints include progression-free survival (PFS) and event rate of atrial fibrillation or flutter; other secondary endpoints include duration of response, overall survival, and incidence of adverse events. The study is ongoing, with pre-specified final analysis of ORR superiority by IRC assessment and formal analysis of PFS when the target number of events is reached. Results are expected in 2022.
About BRUKINSA
BRUKINSA is a small molecule inhibitor of Bruton’s tyrosine kinase (BTK) discovered by BeiGene scientists that is currently being evaluated globally in a broad clinical program as a monotherapy and in combination with other therapies to treat various B-cell malignancies. Because new BTK is continuously synthesized, BRUKINSA was specifically designed to deliver complete and sustained inhibition of the BTK protein by optimizing bioavailability, half-life, and selectivity. With differentiated pharmacokinetics compared to other approved BTK inhibitors, BRUKINSA has been demonstrated to inhibit the proliferation of malignant B cells within a number of disease relevant tissues.
BRUKINSA is approved in the following indications and regions:
- For the treatment of mantle cell lymphoma (MCL) in adult patients who have received at least one prior therapy (United States, November 2019)*;
- For the treatment of MCL in adult patients who have received at least one prior therapy (China, June 2020)**;
- For the treatment of chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL) in adult patients who have received at least one prior therapy (China, June 2020)**;
- For the treatment of relapsed or refractory MCL (United Arab Emirates, February 2021); and
- For the treatment of Waldenström’s macroglobulinemia (WM) in adult patients (Canada, March 2021).
To-date, more than 30 marketing authorization applications in multiple indications have been submitted outside of the United States and China, covering countries in the European Union and more than 20 other countries.
* This indication was approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
** This indication was approved under conditional approval. Complete approval for this indication may be contingent upon results from ongoing randomized, controlled confirmatory clinical trials.
IMPORTANT U.S. SAFETY INFORMATION FOR BRUKINSA (ZANUBRUTINIB)
Warnings and Precautions
Hemorrhage
Fatal and serious hemorrhagic events have occurred in patients with hematological malignancies treated with BRUKINSA monotherapy. Grade 3 or higher bleeding events including intracranial and gastrointestinal hemorrhage, hematuria and hemothorax have been reported in 2% of patients treated with BRUKINSA monotherapy. Bleeding events of any grade, including purpura and petechiae, occurred in 50% of patients treated with BRUKINSA monotherapy.
Bleeding events have occurred in patients with and without concomitant antiplatelet or anticoagulation therapy. Co-administration of BRUKINSA with antiplatelet or anticoagulant medications may further increase the risk of hemorrhage.
Monitor for signs and symptoms of bleeding. Discontinue BRUKINSA if intracranial hemorrhage of any grade occurs. Consider the benefit-risk of withholding BRUKINSA for 3-7 days pre- and post-surgery depending upon the type of surgery and the risk of bleeding.
Infections
Fatal and serious infections (including bacterial, viral, or fungal) and opportunistic infections have occurred in patients with hematological malignancies treated with BRUKINSA monotherapy. Grade 3 or higher infections occurred in 23% of patients treated with BRUKINSA monotherapy. The most common Grade 3 or higher infection was pneumonia. Infections due to hepatitis B virus (HBV) reactivation have occurred.
Consider prophylaxis for herpes simplex virus, pneumocystis jiroveci pneumonia and other infections according to standard of care in patients who are at increased risk for infections. Monitor and evaluate patients for fever or other signs and symptoms of infection and treat appropriately.
Cytopenias
Grade 3 or 4 cytopenias, including neutropenia (27%), thrombocytopenia (10%), and anemia (8%) based on laboratory measurements, were reported in patients treated with BRUKINSA monotherapy.
Monitor complete blood counts during treatment and treat using growth factor or transfusions, as needed.
Second Primary Malignancies
Second primary malignancies, including non-skin carcinoma, have occurred in 9% of patients treated with BRUKINSA monotherapy. The most frequent second primary malignancy was skin cancer (basal cell carcinoma and squamous cell carcinoma of skin), reported in 6% of patients. Advise patients to use sun protection.
Cardiac Arrhythmias
Atrial fibrillation and atrial flutter have occurred in 2% of patients treated with BRUKINSA monotherapy. Patients with cardiac risk factors, hypertension, and acute infections may be at increased risk. Grade 3 or higher events were reported in 0.6% of patients treated with BRUKINSA monotherapy. Monitor signs and symptoms for atrial fibrillation and atrial flutter and manage as appropriate.
Embryo-Fetal Toxicity
Based on findings in animals, BRUKINSA can cause fetal harm when administered to a pregnant woman. Administration of zanubrutinib to pregnant rats during the period of organogenesis caused embryo-fetal toxicity, including malformations at exposures that were 5 times higher than those reported in patients at the recommended dose of 160 mg twice daily. Advise women to avoid becoming pregnant while taking BRUKINSA and for at least 1 week after the last dose. Advise men to avoid fathering a child during treatment and for at least 1 week after the last dose. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
Adverse Reactions
The most common adverse reactions in > 10% of patients who received BRUKINSA were neutrophil count decreased (53%), platelet count decreased (39%), upper respiratory tract infection (38%), white blood cell count decreased (30%), hemoglobin decreased (29%), rash (25%), bruising (23%), diarrhea (20%), cough (20%), musculoskeletal pain (19%), pneumonia (18%), urinary tract infection (13%), hematuria (12%), fatigue (11%), constipation (11%), and hemorrhage (10%). The most frequent serious adverse reactions were pneumonia (11%) and hemorrhage (5%).
Of the 118 patients with MCL treated with BRUKINSA, 8 (7%) patients discontinued treatment due to adverse reactions in the trials. The most frequent adverse reaction leading to treatment discontinuation was pneumonia (3.4%). One (0.8%) patient experienced an adverse reaction leading to dose reduction (hepatitis B).
Drug Interactions
CYP3A Inhibitors: When BRUKINSA is co-administered with a strong CYP3A inhibitor, reduce BRUKINSA dose to 80 mg once daily. For co-administration with a moderate CYP3A inhibitor, reduce BRUKINSA dose to 80 mg twice daily.
CYP3A Inducers: Avoid co-administration with moderate or strong CYP3A inducers.
Specific Populations
Hepatic Impairment: The recommended dose of BRUKINSA for patients with severe hepatic impairment is 80 mg orally twice daily.
INDICATION
BRUKINSA is a kinase inhibitor indicated for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy.
This indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
Please see full U.S. Prescribing Information at www.beigene.com/PDF/BRUKINSAUSPI.pdf and Patient Information at www.beigene.com/PDF/BRUKINSAUSPPI.pdf.
BeiGene Oncology
BeiGene is committed to advancing best and first-in-class clinical candidates internally or with like-minded partners to develop impactful and affordable medicines for patients across the globe. We have a growing R&D team of approximately 2,300 colleagues dedicated to advancing more than 90 clinical trials involving more than 13,000 patients and healthy volunteers. Our expansive portfolio is directed by a predominantly internalized clinical development team supporting trials in more than 40 countries. Hematology-oncology and solid tumor targeted therapies and immuno-oncology are key focus areas for the Company, with both mono- and combination therapies prioritized in our research and development. The Company currently markets three medicines discovered and developed in our labs: BTK inhibitor BRUKINSA in the United States, China, Canada, and additional international markets; and non-FC-gamma receptor binding anti-PD-1 antibody tislelizumab and PARP inhibitor pamiparib in China.
BeiGene also partners with innovative companies who share our goal of developing therapies to address global health needs. We commercialize a range of oncology medicines in China licensed from Amgen and Bristol Myers Squibb. We also plan to address greater areas of unmet need globally through our collaborations including with Amgen, Bio-Thera, EUSA Pharma, Mirati Therapeutics, Seagen, and Zymeworks. BeiGene has also entered into a collaboration with Novartis Pharma AG granting Novartis rights to develop, manufacture, and commercialize tislelizumab in North America, Europe, and Japan.
About BeiGene
BeiGene is a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide. With a broad portfolio of more than 40 clinical candidates, we are committed to expediting the development of our diverse pipeline of novel therapeutics through collaborations or our own internal capabilities, with the aspirational goal of radically improving access to medicines for two billion more people by 2030. BeiGene is a headquarter-less company by design, with a growing global team of approximately 6,000 colleagues across five continents. To learn more about BeiGene, please visit www.beigene.com and follow us on Twitter at @BeiGeneGlobal.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws, including statements regarding the results from the interim analysis of the Phase 3 ALPINE trial, the potential clinical benefits and advantages of BRUKINSA compared to other BTK inhibitors, expected timelines for the final analysis of the ALPINE trial and topline results from the Phase 3 SEQUOIA trial, BeiGene's plan for the advancement, and anticipated clinical development, regulatory milestones and commercialization of BRUKINSA, and BeiGene’s plans, commitments, aspirations, and goals under the headings “BeiGene Oncology” and “About BeiGene”. Actual results may differ materially from those indicated in the forward-looking statements as a result of various important factors, including the risk that preliminary data from the interim analysis of the Phase 3 ALPINE trial may differ at the final analysis; the risk that interim and/or final results of the ALPINE trial will not support filings for regulatory approvals of BRUKINSA for the treatment of patients with CLL, and the timing of any such filings and potential approvals; clinical data continue to support a risk-benefit profile for BRUKINSA; BeiGene's ability to demonstrate the efficacy and safety of its drug candidates; the clinical results for its drug candidates, which may not support further development or marketing approval; actions of regulatory agencies, which may affect the initiation, timing and progress of clinical trials and marketing approval; BeiGene's ability to achieve commercial success for its marketed medicines and drug candidates, if approved; BeiGene's ability to obtain and maintain protection of intellectual property for its medicines and technology; BeiGene's reliance on third parties to conduct drug development, manufacturing and other services; BeiGene’s limited experience in obtaining regulatory approvals and commercializing pharmaceutical products and its ability to obtain additional funding for operations and to complete the development and commercialization of its drug candidates and achieve and maintain profitability; the impact of the COVID-19 pandemic on the BeiGene’s clinical development, regulatory, commercial, and other operations, as well as those risks more fully discussed in the section entitled “Risk Factors” in BeiGene’s most recent quarterly report on Form 10-Q as well as discussions of potential risks, uncertainties, and other important factors in BeiGene's subsequent filings with the U.S. Securities and Exchange Commission. All information in this press release is as of the date of this press release, and BeiGene undertakes no duty to update such information unless required by law.
References
- American Cancer Society. Cancer Facts & Figures 2021. Atlanta; American Cancer Society; 2021. Available here: Cancer Facts and Figures 2021
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017. JAMA Oncol. 2019;5(12):1749-1768.
- National Cancer Institute. Chronic Lymphocytic Leukemia Treatment (PDQ®)–Patient Version. Available here: Chronic Lymphocytic Leukemia Treatment (PDQ®)–Patient Version
- Haselager MV et al. Proliferative Signals in Chronic Lymphocytic Leukemia; What Are We Missing? Front Oncol. 2020; 10: 592205.
- Cancer Support Community. Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Available here: https://www.cancersupportcommunity.org/chronic-lymphocytic-leukemiasmall-lymphocytic-lymphoma.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210611005105/en/
Contacts
Investor Contact
Craig West
+1 857-302-5189
ir@beigene.com
Media Contact
Liza Heapes or Vivian Ni
+1 857-302-5663 or +1 857-302-7596
media@beigene.com














