Consensus symposium and multiple poster abstracts demonstrate efficacy of fish-skin grafts in wound treatment and reconstructive surgery
SAWC – Kerecis today announced the publication of a multi-disciplinary clinical consensus about the utilization of fish skin grafts on complex wounds. The consensus paper will be presented at a CME/CPME/CE-accredited symposium, which will take place Saturday, November 4, from 11:30 am to 12:30 pm in Milano VII-VIII, Caesars Palace, Las Vegas at the SAWC wound conference. Pre-registration is not required for conference attendees. Kerecis, which is pioneering the use of sustainably sourced fish skin and fatty acids in cellular therapy and tissue regeneration and protection, is exhibiting at booth 517.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20231103279415/en/
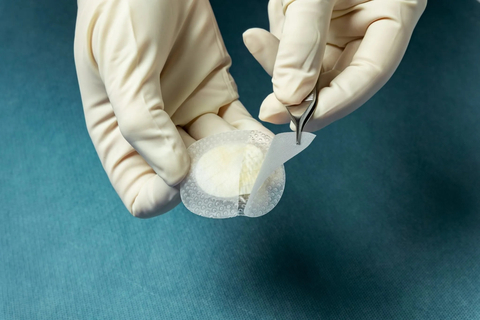
Kerecis' fish-skin grafts are available in various shapes and sizes, such as Kerecis Shield, as shown. (Photo: Business Wire)
The symposium, which is entitled “The CODsensus: Real World Results for the Use of Intact Fish Skin in the Treatment of VLUs, DFUs, and Atypical Wounds,” will present the multi-disciplinary clinical consensus on using fish skin grafts for wound treatment, as well as discuss the best practices for treating various lower extremity wound types. The presenters are John C. Lantis II, MD (Mount Sinai West and the Icahn School of Medicine, New York, New York); Caroline E. Fife, MD (Intellicure, LLC, The Woodlands, Texas); and Eric J. Lullove, DPM, CWSP, FAPWH(c), DABLES (West Boca Center for Wound Healing, Coconut Creek, Florida).
The SAWC conference accepted 36 investigator-initiated poster abstracts that discuss the use of fish-skin grafts in various types of wounds and reconstructive operations. The abstracts are on display in the conference poster room and will be highlighted at the conference poster reception that will take place Saturday, November 4, from 5:00 pm to 6:30 pm in the Julius XII room.
“The clinical consensus described in the symposium represents another milestone in the ongoing acceptance of our Arctic-sourced fish skin evolving to become the standard of care for complicated wounds and tissue trauma,” said Fertram Sigurjonsson, founder and CEO of Kerecis. “The products are manufactured in our factory in Northwest Iceland from catch from a sustainable fish stock in the pristine Icelandic waters.”
About Kerecis
Kerecis develops products from fish skin and fatty acids for cellular therapy, tissue regeneration and protection. When grafted onto damaged human tissue or implanted, the patented material supports the body’s own processes to heal and regenerate. Because no disease-transfer risk exists between cold-water fish and humans, the Kerecis fish skin is only gently processed and retains its similarity to human tissue. The gentle processing preserves the skin’s original three-dimensional structure, maintaining its inherent natural strength, complexity and molecules (such as fatty acids). Clinical studies have found that the Kerecis products heal wounds faster than competing products. Kerecis is the only approved manufacturer of medical devices containing intact fish skin globally.
Kerecis is the fastest-growing and one of the top five companies in the U.S. biologics-skin and dermal-substitute market, according to SmartTRAK Business Intelligence. Kerecis’s expanding product portfolio includes SurgiBind®/SurgiClose®, which are used for reconstructive surgery in hospital operating rooms; GraftGuide®, which is mostly sold to burn centers; and MariGen® and Shield™, which are sold to out-patient healthcare facilities to treat multiple wound types including diabetic wounds and wounds resulting from Mohs surgery.
Kerecis is committed to the United Nations Sustainable Development Goals. The fish skin used in Kerecis products derives from wild and sustainable fish stock caught in pristine Icelandic waters and processed with 100% renewable energy in the town of Isafjordur, close to the Arctic Circle. Kerecis is part of Coloplast, the leading global supplier of intimate healthcare products. For more information, visit https://www.kerecis.com.
Trademarks and registered trademarks are the property of their respective owners.
View source version on businesswire.com: https://www.businesswire.com/news/home/20231103279415/en/
Contacts
Kay Paumier
Communications Plus
kay@communicationsplus.net
408-370-1243
Mobile: 408-806-1177














