- Can-Fite Signed agreement with Fondazione Telethon for co-development of Piclidenoson for the treatment of Lowe syndrome an estimated $100 M market in the U.S. only with no drug available
- FDA & EMA approvals for rare genetic diseases are fast and require clinical studies with small number of patients
- Dr. Antonella De Matteis, Professor of Biology, Department of Molecular Medicine and Medical Biotechnology at the University of Naples Federico II, and Program Coordinator of the Cell Biology and Disease Mechanisms at The Telethon Institute of Genetics and Medicine (TIGEM) in Italy
Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small molecule drugs that address inflammatory, cancer and liver diseases, announced today it plans to develop its lead drug candidate, Piclidenoson, for the treatment of Lowe Syndrome based on recent efficacy findings by Dr. Antonella De Matteis, Professor of Biology, Department of Molecular Medicine and Medical Biotechnology at the University of Naples Federico II, and Program Coordinator of the Cell Biology and Disease Mechanisms at The Telethon Institute of Genetics and Medicine (TIGEM) in Italy. Can-Fite and Fondazione Telethon have signed an agreement outlining their collaboration for the development of Piclidenoson for the treatment of Lowe Syndrome, a high medical need with no drug available. Fondazione Telethon is an Italian nonprofit organization whose mission is to advance biomedical research toward the diagnosis, cure, and prevention of genetic diseases, and it established TIGEM in 1994.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230824833299/en/
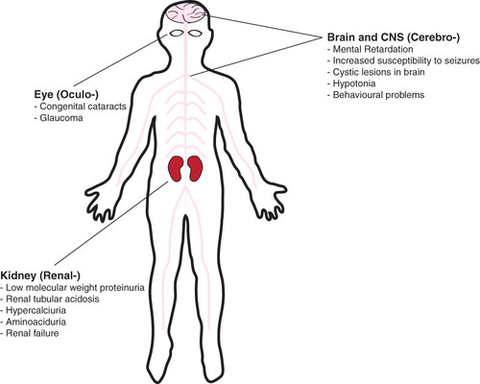
Lowe Syndrome, also known as oculo-cerebro-renal syndrome (OCRL), an X-linked genetic condition occurring almost exclusively in males, is a multisystem disorder characterized by vision problems including clouding of the lenses of the eyes (cataracts) that are present at birth, kidney problems that usually develop in the first year of life, and brain abnormalities associated with intellectual disabilities, and a life span that rarely exceeds 40 years. Lowe Syndrome prevalence is estimated at approximately 1 in 500,000. (Graphic: Business Wire)
Lowe Syndrome, also known as oculo-cerebro-renal syndrome (OCRL), an X-linked genetic condition occurring almost exclusively in males, is a multisystem disorder characterized by vision problems including clouding of the lenses of the eyes (cataracts) that are present at birth, kidney problems (consisting in urinary loss of proteins and solutes) that usually develop in the first year of life, and brain abnormalities associated with intellectual disabilities, and a life span that rarely exceeds 40 years. Lowe Syndrome prevalence is estimated at approximately 1 in 500,000.
“Having tested thousands of compounds in search of a treatment for Lowe Syndrome, Piclidenoson is the only compound we’ve found to date that has shown to be effective in pre-clinical studies. Importantly, we observed that Piclidenoson treatment in mouse models of Lowe syndrome leads to a significant decrease of the urinary loss of proteins in diseased animals,” Dr. De Matteis stated. “We chose to investigate Piclidenoson based on the availability of extensive scientific data showing its excellent safety, coupled with efficacy in this disease which involves renal, cerebral, and ocular manifestations.”
Can-Fite Chairman Dr. Pnina Fishman commented, “We are hopeful that Piclidenoson can offer a much needed treatment for infants, children, and young people living with Lowe Syndrome. Based on Piclidenoson’s proven safety profile in clinical trials to date, and because Lowe is a rare disease in dire need of treatment, we plan to move into an advanced stage clinical study which may open a path to approval. Dr. De Matteis and her team have made an impactful discovery with Piclidenoson and we look forward to working with her and Fondazione Telethon.”
Can-Fite estimates the total addressable market for the treatment of Lowe Syndrome at approximately $100 million in the U.S. alone, based on incidence and cost of treatment for other rare genetic pediatric syndromes. The Company is not aware of any drug candidates currently in development for the systemic treatment of Lowe Syndrome.
As a rare genetic pediatric disorder, Lowe Syndrome may qualify for Orphan Drug Designation by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), granting special development status to accelerate development at a reduced cost. The FDA’s Orphan Drug designation grants 7 years of market exclusivity, tax credits, waiver of the Prescription Drug User Fee Amendments fee (currently $3.1 million in 2022), and access to additional grants and support from the FDA. Piclidenoson for the treatment of Lowe Syndrome may also qualify for the FDA’s Rare Pediatric Disease Priority Review Voucher, granting an accelerated review process for marketing approval.
About Fondazione Telethon
Fondazione Telethon ETS is one of the main Italian biomedical charities, founded in 1990 on the initiative of a group of patients suffering from muscular dystrophy. Its mission is to achieve the cure of rare genetic diseases through scientific research of excellence, selected according to the best practices shared internationally. Through a unique method in the Italian panorama, it follows the entire "research chain" dealing with fundraising, selection and funding of projects and the research activity itself carried out in the centers and laboratories of the Foundation. Telethon also develops collaborations with public health institutions and pharmaceutical industries to translate the results of research into therapies accessible to patients. Since its foundation, Telethon has invested more than 660 million euros in research, has funded 2,960 projects with 1,720 researchers involved and 630 diseases studied. To date, thanks to Fondazione Telethon, the first gene therapy with stem cells in the world has been made available, thanks to the collaboration with the pharmaceutical industry. This therapy is intended for the treatment of ADA-SCID, a severe immunodeficiency that compromises the body's defenses from birth. In 2023, Fondazione Telethon became responsible for the production and distribution of the drug to eligible patients in the European Union
Another gene therapy resulting from Telethon research made available is the one for a serious neurodegenerative disease, metachromatic leukodystrophy. This therapeutic approach is in an advanced stage of development for another immunodeficiency, Wiskott-Aldrich syndrome. Other diseases on which the gene therapy developed by Telethon researchers has been evaluated in patients are beta thalassemia and two metabolic diseases of childhood, mucopolysaccharidosis type 6 and type 1. In addition, within the Telethon institutes a targeted therapeutic strategy is being studied or developed for other genetic diseases, such as hemophilia or various hereditary vision defects. In parallel, the study of basic mechanisms and potential therapeutic approaches for diseases still unanswered continues in all laboratories funded by Telethon.
About Piclidenoson
Piclidenoson is a novel, first-in-class, A3 adenosine receptor agonist (A3AR) small molecule, orally bioavailable drug with an excellent safety profile demonstrating evidence of efficacy in Phase II and Phase III clinical studies. The drug’s mechanism of action entails inhibition of the inflammatory cytokines interleukin 17 and 23 (IL-17 and IL-23) and the induction of apoptosis of patients’ skin cell keratinocytes involved with the disease pathogenicity.
About Can-Fite BioPharma Ltd.
Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CFBI) is an advanced clinical stage drug development Company with a platform technology that is designed to address multi-billion dollar markets in the treatment of cancer, liver, and inflammatory disease. The Company's lead drug candidate, Piclidenoson recently reported topline results in a Phase III trial for psoriasis. Can-Fite's liver drug, Namodenoson, is being evaluated in a Phase IIb trial for the treatment of non-alcoholic steatohepatitis (NASH), and enrollment is expected to commence in a Phase III trial for hepatocellular carcinoma (HCC), the most common form of liver cancer. Namodenoson has been granted Orphan Drug Designation in the U.S. and Europe and Fast Track Designation as a second line treatment for HCC by the U.S. Food and Drug Administration. Namodenoson has also shown proof of concept to potentially treat other cancers including colon, prostate, and melanoma. CF602, the Company's third drug candidate, has shown efficacy in the treatment of erectile dysfunction. These drugs have an excellent safety profile with experience in over 1,500 patients in clinical studies to date. For more information please visit: www.can-fite.com.
Forward-Looking Statements
This press release may contain forward-looking statements, about Can-Fite’s expectations, beliefs or intentions regarding, among other things, its product development efforts, business, financial condition, results of operations, strategies or prospects. All statements in this communication, other than those relating to historical facts, are “forward looking statements”. Forward-looking statements can be identified by the use of forward-looking words such as “believe,” “expect,” “intend,” “plan,” “may,” “should” or “anticipate” or their negatives or other variations of these words or other comparable words or by the fact that these statements do not relate strictly to historical or current matters. Forward-looking statements relate to anticipated or expected events, activities, trends or results as of the date they are made. Because forward-looking statements relate to matters that have not yet occurred, these statements are inherently subject to known and unknown risks, uncertainties and other factors that may cause Can-Fite’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Important factors that could cause actual results, performance or achievements to differ materially from those anticipated in these forward-looking statements include, among other things, our history of losses and needs for additional capital to fund our operations and our inability to obtain additional capital on acceptable terms, or at all; uncertainties of cash flows and inability to meet working capital needs; the initiation, timing, progress and results of our preclinical studies, clinical trials and other product candidate development efforts; our ability to advance our product candidates into clinical trials or to successfully complete our preclinical studies or clinical trials; our receipt of regulatory approvals for our product candidates, and the timing of other regulatory filings and approvals; the clinical development, commercialization and market acceptance of our product candidates; our ability to establish and maintain strategic partnerships and other corporate collaborations; the implementation of our business model and strategic plans for our business and product candidates; the scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates and our ability to operate our business without infringing the intellectual property rights of others; competitive companies, technologies and our industry; risks related to the COVID-19 pandemic and the Russian invasion of Ukraine; risks related to not satisfying the continued listing requirements of NYSE American; and statements as to the impact of the political and security situation in Israel on our business. More information on these risks, uncertainties and other factors is included from time to time in the “Risk Factors” section of Can-Fite’s Annual Report on Form 20-F filed with the SEC on March 24, 2022 and other public reports filed with the SEC and in its periodic filings with the TASE. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Can-Fite undertakes no obligation to publicly update or review any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by any applicable securities laws.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230824833299/en/
Contacts
Can-Fite BioPharma
Motti Farbstein
info@canfite.com
+972-3-9241114














