Delcath Systems, Inc. (Nasdaq: DCTH) (“Company” or “Delcath”), an interventional oncology company focused on the treatment of primary and metastatic liver cancers, today announced the publication of an independent study conducted by investigators at the University Hospital of Leipzig, Germany, in the European Society for Medical Oncology journal of Gastrointestinal Oncology. The study, titled “Hepatic chemosaturation with melphalan in patients with primary or secondary liver tumors with or without extrahepatic tumor manifestation,” highlights the efficacy and safety of repeated chemosaturation treatments using Delcath’s CHEMOSAT® Hepatic Delivery System.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240826877468/en/
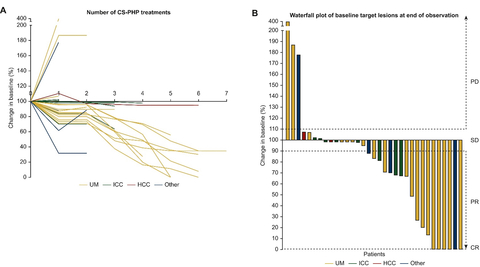
Figure 1: Individual changes in tumor sizes during CHEMOSAT treatment. (A) Individual changes in tumor sizes during CHEMOSAT treatment. On the y-axis the change in tumor size is indicated according to the baseline lesions. (B) Waterfall plot of changes in the size of target lesions at the end of observation. Dashed lines are thresholds for hepatic PD, hepatic SD, hepatic PR and hepatic CR. CR, complete response; HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocellular carcinoma; other, ciliary body melanoma, angiosarcoma, tonsil and pancreatic carcinoma; PD, progressive disease; PR, partial response; SD, stable disease; UM, uveal melanoma. (Graphic: Business Wire)
Key Findings from the Independent Study:
- Patient Population: This retrospective study evaluated the efficacy of CHEMOSAT in 33 previously treated patients with unresectable intrahepatic metastases from various cancers: uveal melanoma (N=19), cholangiocarcinoma (N=8), hepatocellular carcinoma (N=2), and one patient each with ciliary body melanoma, acinar cell carcinoma, pancreatic cancer, or tonsil cancer (N=4). In addition to hepatic metastases, 7 out of 33 patients also had limited extrahepatic disease, which was found not to significantly impact overall survival.
- Disease Control Rate: The study reported a disease control rate (DCR) of 91%, with 30 out of 33 patients experiencing either objective tumor response or stable disease. Notably, 6 patients (18.2%) achieved complete response (CR) in the liver, including 5 patients with uveal melanoma and 1 patient with cholangiocarcinoma, who received a median of 5 treatment cycles.
-
Hepatic Progression-Free Survival: Median hepatic progression-free survival (hPFS) was 52 weeks across all patients, with particularly strong outcomes for specific cancers:
- 69 weeks (16 months) median hPFS in patients with uveal melanoma.
- 38 weeks (8.5 months) median hPFS in patients with cholangiocarcinoma.
- Importance of Repeated Treatments: The investigators’ approach of using CHEMOSAT in the form of regularly repeated treatment cycles as clinically indicated (Figure 1), similar to systemic chemotherapy, resulted in long-term disease control in the majority of patients and was well tolerated.
- Tolerability and Safety: The safety profile of CHEMOSAT was consistent with published literature. Most patients experienced transient hematological adverse events, which were routinely managed with supportive care. Importantly, no significant liver damage was reported, even in patients who underwent multiple treatment cycles. Treatment was discontinued in 2 patients due to adverse events, and 2 patients withdrew consent during the treatment period.
Dr. Vojislav Vukovic, Chief Medical Officer of Delcath Systems, commented, “The results of this independent study reinforce the potential of our CHEMOSAT Hepatic Delivery System as an essential tool in the management of primary and secondary liver tumors, particularly for patients with limited treatment options. The high rate of disease control observed, even in patients with extrahepatic tumor spread, underscores the importance of continuing to explore and refine this treatment approach in larger, prospective trials.”
The publication is available here.
About Delcath Systems, Inc., HEPZATO KIT and CHEMOSAT
Delcath Systems, Inc. is an interventional oncology company focused on the treatment of primary and metastatic liver cancers. The company's proprietary products, HEPZATO KIT™ (HEPZATO (melphalan) for Injection/Hepatic Delivery System) and CHEMOSAT® Hepatic Delivery System for Melphalan percutaneous hepatic perfusion (PHP), are designed to administer high-dose chemotherapy to the liver while controlling systemic exposure and associated side effects during a PHP procedure.
In the United States, HEPZATO KIT is considered a combination drug and device product and is regulated and approved for sale as a drug by the FDA. HEPZATO KIT is comprised of the chemotherapeutic drug melphalan and Delcath's proprietary Hepatic Delivery System (HDS). The HDS is used to isolate the hepatic venous blood from the systemic circulation while simultaneously filtrating hepatic venous blood during melphalan infusion and washout. The use of the HDS results in loco-regional delivery of a relatively high melphalan dose, which can potentially induce a clinically meaningful tumor response with minimal hepatotoxicity and reduce systemic exposure. HEPZATO KIT is approved in the United States as a liver-directed treatment for adult patients with metastatic uveal melanoma (mUM) with unresectable hepatic metastases affecting less than 50% of the liver and no extrahepatic disease, or extrahepatic disease limited to the bone, lymph nodes, subcutaneous tissues, or lung that is amenable to resection or radiation. Please see the full Prescribing Information, including BOXED WARNING for the HEPZATO KIT.
In Europe, the device-only configuration of the HDS is regulated as a Class III medical device and is approved for sale under the trade name CHEMOSAT Hepatic Delivery System for Melphalan, or CHEMOSAT, where it has been used in the conduct of percutaneous hepatic perfusion procedures at major medical centers to treat a wide range of cancers of the liver.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240826877468/en/
Contacts
Investor Relations:
ICR Westwicke
investorrelations@delcath.com














