Akebia Therapeutics Inc. (NASDAQ: AKBA) is a biopharmaceutical company specializing in developing treatments for chronic kidney diseases (CKD) and anemia management for patients with CKD. The medical sector company addresses unmet needs in the segment. It received FDA approval for its lead candidate, Vafseo, which is used to treat anemia in adult patients on dialysis suffering from chronic kidney disease (CKD). This applies to a total addressable market (TAM) of 500,000 adult patients on dialysis suffering from anemia due to CKD. Vafseo is an oral tablet competing with current treatments like Jesduvroq from GSK plc (NYSE: GSK) and blood transfusions in severe cases.
What is Anemia?
Anemia is a condition triggered by the deficiency of hemoglobin in the red blood cells. Hemoglobin is the protein that transports oxygen from the lungs to the body. A lack of hemoglobin results in organs not receiving enough oxygen, which can cause symptoms like fatigue, pale skin, weakness, dizziness, shortness of breath and rapid heartbeat.
Many types of anemia, like iron and vitamin-deficiency anemia, are caused by a lack of iron to produce red blood cells. Hemolytic anemia forms from the premature destruction of red blood cells from an underlying condition like infections or autoimmune disorders. Aplastic anemia is caused by damage to the bone marrow preventing it from producing red blood cells.
Anemia in CKD Patients
Anemia in CKD patients occurs when the kidneys can't produce enough erythropoietin (EPO), a hormone that stimulates the production of red blood cells in bone marrow. This can occur from kidney disease, inflammatory disorders or cancer. This results in patients having much lower than normal red blood cells, leading to anemia.
The Vafseo Pill Treatment
Akebia's lead therapeutic, Vafseo (vadadustat), treats anemia by mimicking the natural response to low oxygen levels by the body. It belongs to a class of drugs called hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitors (HIF-PHIs). HIF-PHIs are enzymes that are normally produced by healthy kidneys to help regulate HIF. Vafseo increases HIF levels are inhibiting HIF-PH enzymes. Rising HIF levels trigger the natural production of EPO, and as more EPO is produced the bone marrow also increases red blood cell production, which improves hemoglobin levels to alleviate anemia symptoms.
Vafseo Receives FDA Approval
On March 28, 2024, the U.S. Food and Drug Administration (FDA) approved Vafseo tablets for treating anemia with CKD for adult patients with at least 3 months of dialysis. This overturns an earlier denials for marketing approval due to safety concerns. This approval makes Vafseo approved in 37 countries as a once-daily HIF-PH inhibitor pill. This was backed by Akeba's global Phase 3 INNO2VATE program and post-marketing safety data from Japan. Japan approved it in 2000.
The Founder and President of the Renal Support Network, Lori Hartwell, has been suffering from CKD since she was a child and expressed her support, "Anemia is a debilitating condition that significantly impacts our daily lives. It is promising to see the introduction of innovative treatment options for people fighting anemia."
Vafseo Receives FDA Approval
The FDA approval enables Akebia to commercialize Vafseo in the United States with its partner CSL Limited (OTCMKTS: CSLLY). CSL holds the exclusive license to sell Vafseo to third-party dialysis organizations and Fresenius Kidney Care dialysis centers. It competes with GSK plc-owned Jesduvroq, which was approved in February 2023 and is Japan's leading HIF-PHF drug.
Akebia CEO John Butler stated, "With the approval of Vafseo in the U.S., we're proud to deliver an alternative treatment option for the hundreds of thousands of Americans on dialysis who are diagnosed with anemia due to CKD." Butler concluded, “At Akebia we are committed to kidney patients, a dedication that has driven our team to achieve this milestone. We believe this commitment uniquely positions the company to execute a successful launch designed to drive toward a potential new oral standard of care for dialysis patients."
Results for Q4 2023
Prior to the FDA approval, Akebia released its Q4 2023 earnings report on March 14, 2024. The company reported EPS of breakeven, which was 4 cents better than consensus estimates. Revenues grew 1.84% YoY To 56.2 million. The company received a $55 million term loan facility and $26 million in gross proceeds from at-the-market (ATM) stock sales.
Akebia Therapeutics analyst ratings and price targets are at MarketBeat. Akebia’s peers and competitor stocks can be found with the MarketBeat stock screener.
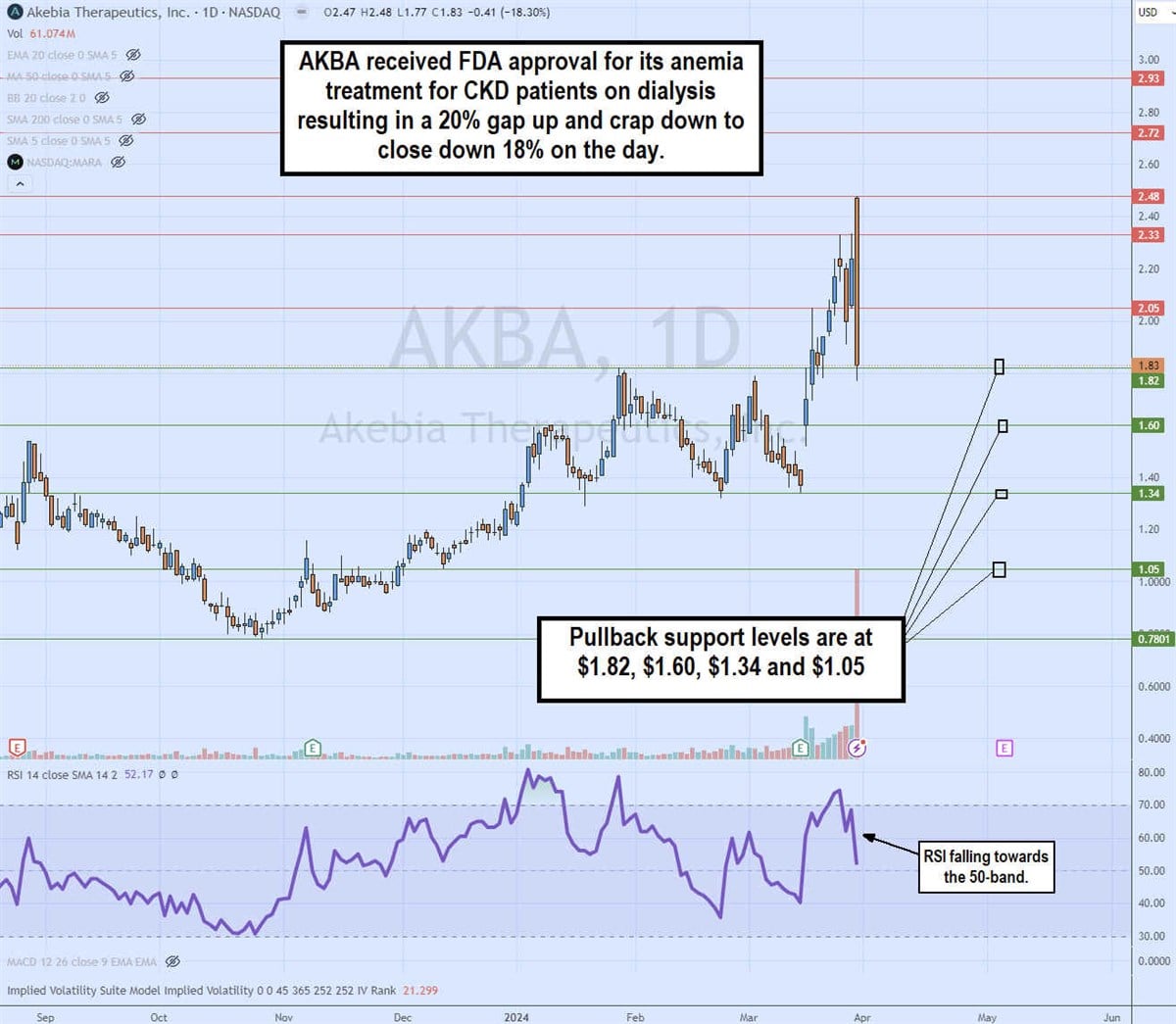
Daily Bearish Engulfing Pattern
The daily candlestick chart on PATH illustrates a bearish engulfing candle pattern. Shares of AKBA surged over 20% on the FDA approval news to $2.48 but formed a gap and crap reaction to close at $1.83, down 18% on the day. The bearish engulfing candle swallowed the range of the 7 preceding candles, which can be viewed as bearish. The daily relative strength index (RSI) took a sharp drop, rejecting the 70-band to fall to the 52-band. Pullback support levels are at $1.82, $1.60, $1.34 and $1.05.













