Provides the Opportunity to Sell SafeBreak® Vascular in International Markets
FAYETTEVILLE, Ark. - Feb. 8, 2023 - PRLog -- Lineus Medical has been granted Certificates of Registration for both EN IS0 13485:2016 and 13485:2016/MDSAP. These certify that the quality management system of Lineus Medical has been registered as conforming to high caliber, international standards. The certified quality management system applies to the design and development, manufacture, and distribution of SafeBreak Vascular.
The certifications were awarded based on passing a rigorous two stage audit conducted by Intertek, an international quality assurance and audit organization. The certificates allow Lineus Medical to enter into international markets with SafeBreak Vascular, the company's flagship product.
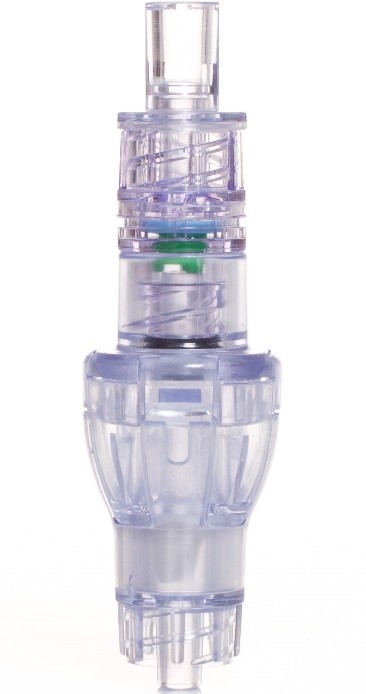
SafeBreak Vascular is a break-away connector that fits into any peripheral IV line on any standard luer connector in the world, although currently it is only cleared for use in the United States. The device is designed to separate when a damaging force is placed on an IV line, therefore reducing irritation of the vein and the chances of the IV dislodging. When the device separates, valves on each side of the device close to stop the flow of medication from the pump and blood flow from the patient's IV catheter. The separated SafeBreak can be replaced with a new, sterile SafeBreak and the IV restarted without the need for another needlestick for the patient. In a 2021 randomized controlled trial, SafeBreak Vascular was shown to reduce IV complications requiring an IV restart by 44%.1
"Obtaining these certifications is huge for the organization and is a testament to our high-Quality standards," Will Armstrong, COO of Lineus Medical stated. "We can now continue executing our strategic plan and expand into regions such as Japan, Korea, Canada, and Europe."
SafeBreak Vascular reduces the failure of peripheral IV lines and the negative cascade of events that occurs when an IV restart is required. Over 235M peripheral IVs are successfully placed in the United States each year and over 2B globally1. Peripheral IVs fail 46% of the time2 before their intended use is complete and cost the US healthcare system over $5B annually1.
ABOUT LINEUS MEDICAL
Lineus Medical is the developer of a patented, break-away technology designed to work with all types of medical tubing. Complications with feeding tubes, IV lines, urinary catheters and other medical tubing may be reduced with a device that breaks into two pieces to protect the patient's medical line. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn or Twitter.
1Data on file.
2Helm, R.E., et al., Accepted but Unacceptable: Peripheral IV Catheter Failure. Journal of Infusion Nursing. 2015; 38(3): 189-203
Contact
Lineus Medical
***@lineusmed.com
Photos: (Click photo to enlarge)![]()


Read Full Story - Lineus Medical® Awarded ISO 13485 and MDSAP Certifications | More news from this source
Press release distribution by PRLog
Lineus Medical® Awarded ISO 13485 and MDSAP Certifications
February 08, 2023 at 13:04 PM EST













